Are you a woman who has undergone breast reconstruction? Have you had complications after your procedure? If so, we need your help!
We’re researchers at [university name] University and we’re conducting a study on the effects of autologous breast reconstruction on women’s mental health. We want to hear from women who have experienced these complications so that we can better understand how they affect our patients and how best to treat them.
We understand that this is a sensitive subject and we will keep all information shared in this survey confidential. Please know that if you choose to participate in the survey, there are no right or wrong answers and you are free to withdraw at any time without penalty.
We’ll also examine nerve pain after latissimus dorsi breast reconstruction and chronic back pain after latissimus dorsi breast reconstruction in this post.
Complications After Autologous Breast Reconstruction
Systematics of Reconstructive Breast Surgery
In general, three treatment situations can be distinguished in surgical breast reconstruction (Figure 1):

Figure 1.
Reconstructive breast surgery: Indications.
- i) Breast-conservation surgery, in which segmental partial defects are reconstructed by local tissue repositioning to avoid contour defects of the breast and to restore the breast shape, size and symmetry to that of the contralateral side. This concept of oncoplastic partial defect reconstruction can also be performed as targeted concept surgery with a few basic surgical techniques that can be learned and taught (targeted oncoplastic breast surgery) (1);
- ii) Skin-saving and nipple-saving techniques of mastectomy require immediate reconstruction, usually as implant reconstruction;
- iii) Interval reconstruction, in a secondary reconstruction of the breast after mastectomy and tumor therapy to restore the physical integrity and improve the quality of life of patients.
Reconstructive Breast Surgery – Risk of Complications
Surgical details, individual patient characteristics, and factors specifically related to the treatment of breast cancer increase the risk for some complication.
Smoking, obesity, and older age are established risk factors (2). Radiotherapy significantly increases the overall risk. Regarding chemotherapy, a meta-analysis showed neither neoadjuvant nor adjuvant chemotherapy affect complication rates after flap- or implant-based breast reconstruction, although wound healing may be delayed (3). Other factors that affect overall complication rates include the type of mastectomy, implant, tissue flap, use of fat grafting, breast size, bilateral surgery, and prior abdominal surgery [for transverse rectus abdominis (TRAM) flap] (4–7).
Patients older than 65 years may have an overall increased risk of perioperative complications compared with younger patients, probably due to medical comorbidities. However, age does not appear to be a predictive factor for reconstructive failure.
The use of acellular matrix (ADMs) is also thought to be associated with an increased risk of complications after implant reconstruction. In a prospective multicenter study, there were no significant differences in major complications, wound infections, or reconstructive failure 2 years after reconstruction when comparing ADM and non-ADM cohorts (8).
Pre-pectoral reconstruction, in which a tissue expander or implant is placed over the pectoralis major muscle, also does not appear to be associated with an increased risk of complications. In a review comparing pre-pectorally placed tissue expander (51 patients, 84 breasts) with subpectoral placement (115 patients, 186 breasts), there was no significant difference in overall complications (17.9% versus 18.8%) (9). Specific complications included hematoma in 2.4%, seroma in 3.6%, cellulitis in 4.8%, and explantation in 1.2%.
Radiotherapy. Expander or implant reconstruction has a significantly increased complication rate in combination with radiotherapy, regardless of when the radiation therapy is delivered (10). A Mastectomy Reconstruction Outcomes Consortium study compared the outcomes of radiation therapy in the setting of autologous or implant-based breast reconstruction (11). Complications occurred in 25.6% of 622 irradiated patients after autologous reconstruction and in 38.9% after implant reconstruction. In 1,625 patients who did not receive radiotherapy, complications occurred in 28.3% after autologous reconstruction and in 21.8% after implant reconstruction.
The consequences of radiation-induced tissue changes include scarring at the implant-tissue junction, capsular contracture, malpositioning, and compromised wound healing, which can lead to dehiscence of the skin incision (Figure 2).

Figure 2.
Implant reconstruction following irradiation of the thoracic wall, with capsular fibrosis, dehiscence and skin necrosis.
Peled et al. evaluated 218 women after mastectomy and expander/implant reconstruction, of whom 85 patients had undergone prior radiation therapy and 133 underwent postmastectomy radiation therapy (12). Patients with prior radiation therapy had more complications after expander reconstruction than after definitive implant reconstruction, including higher explantation rates (15 vs. 5%) and infection rates (20 vs. 8%). However, complication rates after definitive implant reconstruction were significantly higher, nearly four-fold, than in patients with prior radiation therapy.
Direct-to-implant reconstruction in the setting of skin-sparing mastectomy or nipple-sparing mastectomy may have advantages over two-stage expander/implant reconstruction in the setting of radiation. In a review of 1,286 women, Naoum et al. showed that the cumulative incidence of reconstruction complications after direct-to-implant reconstruction was lower (18.2 vs. 36.8%) at a median follow-up of 5.8 years compared with two-stage expander/implant reconstruction (13).
Autologous tissue appears to tolerate radiation-induced tissue damage better than implant-based reconstructions, so the risk of serious complications is not increased. However, flaps may show radiation-induced fat necrosis, fibrosis, atrophy, and flap contracture.
The incidence of late complications (fat necrosis, flap volume loss, flap contracture) is significantly higher in patients with immediate reconstructions that have undergone radiotherapy (14). In a retrospective study of 113 women undergoing radiotherapy after mastectomy and autologous immediate reconstruction, early complications were observed more frequently. Late complications were also more common with autologous immediate reconstruction followed by radiation (32 vs. 44%) (15). In another study comparing autologous TRAM flap reconstruction before (immediate reconstruction) and after radiation (interval reconstruction), late complications were significantly higher in the immediate reconstruction group compared with the interval reconstruction group (88 vs. 9%) (16).
Smoking. Smoking in general is a risk factor for surgical complications because it affects wound healing and blood supply (17). It is an independent risk factor for the development of perioperative complications and is associated with an increased risk of reconstructive failure. In implant-based reconstruction, the incidence of skin necrosis, infection, and implant loss increases with tobacco use. Smoking cessation before surgical intervention is recommended.
In a study of 155 smokers, 76 ex-smokers, and 517 non-smokers who underwent autologous breast reconstruction with TRAM flaps after mastectomy, the risk of complications was evaluated (18). There were no significant differences in complication rates between nonsmokers and former smokers. Therefore, it is active smoking that is considered to increase the risk of complications for reconstruction with a TRAM flap.
Obesity. Obesity is a challenge for breast reconstruction. Obesity increases the risk of adverse events after autologous or implant-based reconstruction. Several studies showed that obese patients have an increased risk of complications and a poorer cosmetic outcome compared to normal-weight women (19–21). In a review of the National Surgical Quality Improvement Program database, obesity [defined as a body mass index (BMI) >40 kg/m2] was found to be associated with a significantly increased risk of perioperative complications compared with nonobese patients. This increase in risk was independent of the surgical method chosen (22). In another study of 404 patients, patients with BMI >40 kg/m2 had significantly increased rates of total flap loss (8.0 vs. 0.5%), delayed wound healing at the abdominal wall (72 vs. 44%), and serious postoperative complications (12 vs. 3%) (7).
Local Surgical Complications
Seroma. Seromas can develop with most types of breast reconstruction. Most seromas occur after removal of a drain. Early occurrence of seroma is more common after implant reconstruction than after flap-based breast reconstruction. Seromas can occur at both the donor and recipient sites of autologous tissue reconstruction. Seroma rates of 12 to 21% have been reported for latissimus dorsi donor sites (23). In a series of deep inferior epigastric perforator (DIEP) flaps, abdominal wall seroma occurred at the donor site in 5% (24). Seroma at the tissue donor site may require puncture.
Significant fluid accumulation in the breast implant pocket (within the capsule) can lead to asymmetry and breast enlargement (25). Seroma formation increases the risk of implant rotation, malposition, and infection. Seroma formation is increased using reconstruction with an acellular dermal matrix (ADM), and ADM adherence may be impaired if the seroma is not treated (26).
Increased seroma formation may be due to increased implant movement, an oversized pocket, as well as increased patient activity (27).
Early-appearing seromas can be punctured percutaneously. Microbial examination of the wound fluid may be useful if infection is suspected. Once drainage is placed, it usually remains until wound flow is ≤30 ml over a 24-hour period.
Late fluid collections around an implant are rare and the etiology is not well understood. These must be assessed according to the patient’s clinical situation and include fluid analysis (including cell counts, cytology, and microbiology) and ultrasound or magnetic resonance imaging. Seromas occurring more than 1 year after surgery require cytological evaluation to rule out breast implant-associated anaplastic large-cell lymphoma (ALCL) (28).
Differential diagnoses for late fluid collection in the breast are:
- Hematoma
- Infection with or without biofilm formation
- Implant rupture
- Inflammation
- Double capsule formation
- Malignancy
- Implant-associated ALCL
If infection and malignancy are ruled out, surgical intervention with capsulectomy, implant removal, and drainage may be required if the fluid collection under the drainage device does not regress. Evidence suggests that fluid collection is most common with textured implants and is caused by friction between the rough surfaces of the textured prosthesis and the capsule. In this case, it may be advisable to remove the textured implant and replace it with a smooth implant (29).
Bleeding and hematoma. Hematomas are a rare complication of breast reconstruction overall (<2%). The affected breast is enlarged and may show bruising. Hematomas usually develop 12-14 hours after surgery. Rarely, hematomas may occur days or weeks later and are associated with minor injury or trauma to the breast. Data published by Collins et al. showed an incidence of postoperative hematoma of 0.92% in 3,474 implant procedures (30). Late hematoma is associated with trauma, coagulation disorders, hyperactivity, and the use of intraoperative corticosteroids. Location, incision and implant type are not factors associated with increased hematoma formation. Careful intraoperative hemostasis usually prevents hematoma formation.
Medications associated with an increased bleeding tendency, such as antiplatelet agents, anticoagulants, or androgenic hormones, should be stopped preoperatively.
Treatment of a hematoma includes its evacuation and hemostasis. Capsular contracture is a common consequence of hematoma formation (31).
Skin necrosis. Skin necrosis is a complication that should be explained in detail prior to surgery. The increased use of nipple-sparing mastectomy in immediate reconstruction has led to an increasing incidence (32).
Several studies showed an association of skin necrosis and nipple-sparing mastectomy (32, 33). This can be attributed to hypoperfusion of the nipple-areolar complex. Depending on the study, the incidence was found to range from 0 to 48% (34). Increased BMI, tobacco use, and prior breast irradiation increase the risk of skin flap necrosis (17, 21). Komorowska-Timek et al. showed that the use of indocyanine green perfusion significantly reduced the incidence of overall complications and reduced the risk of skin flap necrosis during mastectomy from 15.1 to 4% (35).
To reduce skin flap necrosis during mastectomy, preservation of the subdermal plexus, atraumatic skin treatment during mastectomy, and minimization of thermal damage are essential. If swelling, discoloration, or signs of ischemia occur postoperatively, removing volume added to the expander or avoiding pressure with external bandages may be appropriate.
If the area of necrosis is small and closure is possible without excessive tension, debridement with primary closure should be performed. If the skin flap loss is larger, a more conservative approach can be taken if the expander is protected by muscle coverage or ADM and there is no evidence of infection (Figure 3).

Figure 3.
Nipple necrosis with conservative treatment after nipple-sparing mastectomy and implant reconstruction.
Flap complications. For autologous tissue reconstruction of the breast, pedicled flaps and free perforator flaps can be used. Depending on the donor site and surgical technique, different complications may occur. The main complications of autologous tissue reconstruction are flap and fat tissue necrosis, hernia, and lower abdominal flaccidity.
The use of latissimus dorsi flap reconstruction, which is usually performed in combination with expander/implant reconstruction to achieve a necessary reconstruction volume, has declined significantly in recent years (36). The reason for this in the case of whole-breast reconstruction is, among other things, the limitation of shoulder-arm morbidity.
Individual anatomy, as well as experience with autologous reconstruction procedures, have a significant impact on complication rates of the procedure, which may include complete flap loss because of necrosis.
A retrospective analysis of the National Surgical Quality Improvement Program database of 3,296 patients who underwent autologous breast reconstruction found that complication rates with free perforator flaps, compared with pedicled TRAM flap and latissimus dorsi flaps, were significantly higher (19.4 vs. 13.4 vs. 7.1%) (37). In autologous reconstruction with free perforator flap, the reoperation rate (15.6 vs. 9.9 vs. 5.7%) and flap failure (5.7 vs. 3.4 vs. 3%) were higher compared with pedicled reconstructions. However, the differences were not significant. Overall, flap failure and reoperation rates are less than 2% and 5%, respectively, when reconstructions are performed by experienced operators.
The data of Gill et al. demonstrate the incidence of partial flap necrosis to be 2.5% and complete flap necrosis 0.5%; 5.9% of patients required reoperation. The main cause was venous congestion (24).
Flap necrosis/loss. Flap failure is a rare complication in breast reconstruction and occurs when the blood supply to the flap is not guaranteed. This can occur due to both arterial and venous occlusion. Overall, pedicled flaps, such as the latissimus dorsi and TRAM flaps, in which the blood supply is not reconnected, are associated with lower rates of necrosis (<1%) than free flaps, such as the DIEP flap (2 to 5%).
When total flap loss occurs (≤1%), surgery must be performed to remove the necrotic tissue and reconstruct the breast. Partial flap loss limited to small areas of skin can usually be treated conservatively. Inferior perfusion of the fat region of the flap can lead to fat necrosis and loss of the flap. The consecutive tissue fibrosis and calcification may feel like a tumor on postoperative physical examination.
Some studies show that fat necrosis is more common in DIEP flaps compared with TRAM flaps. In their study, Chun et al. examined 105 women with bilateral pedicled TRAM flaps and 58 women with bilateral DIEP flap reconstructions. The DIEP flap cohort had significantly higher rates of partial skin loss, wound dehiscence, and fat necrosis (5). Gill et al. described flap problems in approximately 6% of patients who required surgery (24). Partial flap loss occurred in 2.5% and complete flap loss in fewer than 1% of cases; 13% of patients showed fat necrosis (risk factors were smoking and radiotherapy after reconstruction).
Complications associated with donor tissue. Obesity, active smoking, collagen vascular disease, diabetes, radiation therapy after mastectomy, and the presence of certain abdominal scars increase the risk of wound healing problems at the donor site. Nelsen et al. studied abdominal wall functionality and strength after autologous breast reconstruction with free abdominal flaps (DIEP, TRAM, and SIEA flaps). Fifty-one patients were included with a mean follow-up of 8.1 years (38). Reconstruction with free flaps was associated with improved long-term quality of life in terms of physical and mental health. It showed only minor functional impairment related to the choice of surgical procedure.
Hernia. Hernias are defined as fascial defects. These may occur after autologous reconstruction from the abdominal wall and are related to the degree of injury the procedure. Chun et al. demonstrated that the rate of abdominal wall herniation was similar using bilateral pedicled TRAM flaps and bilateral DIEP flaps (5). The incidence of hernia in reconstructions using DIEP flaps was 0.7% (24).
Minor abdominal wall weaknesses are often asymptomatic. Prominent hernias often require surgical repair in form of duplication of the anterior rectus sheath or reinforcement with mesh. A mesh-free three-layer closure of the fascial defect is recommended for a unilaterally pedicled TRAM (Figure 4).

Figure 4.
Prophylaxis of hernias by meshfree closure of donor defect after transverse rectus abdominis flap (three layers).
Implant-based Complications
Capsular contracture and implant failure are common complications of breast reconstruction with implants and expanders (e.g., rupture, deflation, malposition).
Capsular contracture. Capsular contracture is a common risk in breast implant reconstruction. A thin capsule of connective tissue forms around the breast implant and does not usually cause symptoms. If the capsule is thickened or calcified, it can cause breast pain, hypersensitivity, and distortion (39). Most capsular contractures occur within 12 months after surgery. The use of an ADM in implant reconstructions may reduce the incidence of capsular contracture.
Vardanian et al. compared 123 patients with ADM and 80 without ADM reconstruction. The capsular contracture rate with ADM was 3.8% and without ADM was 19.4% (40). Several factors can contribute to the development of capsular contracture. These include size of the implant, the patient’s scarring tendency, and circulating bacteria.
Although poorly studied, capsular contracture is assumed to be due to low-grade subclinical bacterial infection and the formation of a bacterial biofilm (39). Chronic inflammatory process may also be a possible reason for capsular contracture (39). Capsular contractures occur much less frequently in nonsmokers (8% in smokers vs. 3% in nonsmokers) (41). Hematomas can also lead to capsular contractures (30).
Performing radiation may increase the risk of capsular contracture in breast reconstruction (compared to augmentation). Jagsi et al. showed that capsular contracture developed in only 3% of female patients but it occurred in four out of five cases of previously irradiated patients (11). Regarding the influence of chemotherapy on capsular contracture, no definitive results are available. By using implants with a textured surface instead of a smooth surface, capsular contracture rates might be reduced.
Lower rates of capsular contracture are associated with partial or complete submuscular or subfascial implant placement. In a multicenter study of more than 500 patients, subglandular (compared with submuscular) implant placement resulted in an almost eight-fold increase in the risk of capsular contracture (42).
Nevertheless, the epipectoral plane of implantation is being used with increasing frequency (43). This preserves the integrity of the pectoralis major muscle and eliminates the need for additional mesh materials and acellular matrix in the caudal pole of the breast. There is no distortion during contraction of the pectoralis major muscle (‘jumping breast’) (44). The foreign body sensation is also less pronounced. In the case of significant capsular fibrosis with the need for surgical revision, we recommend conversion to autologous reconstruction (DIEP).
Classification of capsular fibrosis. The Baker classification (45) is used for assessing the severity of breast implant capsules:
- Baker I: The breast is soft with no palpable capsule and looks natural.
- Baker II: The breast is slightly firmer with a palpable capsule, but looks normal.
- Baker III: Breast is firm with a slightly palpable capsule and does not look visually normal.
- Baker IV: The breast is hard, cold, painful, and markedly distorted.
Baker contractures of grades III and IV are generally classified as complications. Baker grades I and II are generally not included in complication rates and treatment recommendations.
Treatment of capsular fibrosis. A surgical approach should be taken for Baker III and IV capsules (capsulotomy, capsulectomy, implant exchange).
Open capsulotomy involves internal circumferential and longitudinal incisions through the capsule. This leaves the capsule in place on the tissue but results in widening of the implant pocket and improves implant deformation. During capsulectomy, the affected scar tissue and capsule are removed. Although open capsulotomy may improve symptoms in the short term, the recurrence rate is high (46).
In the case of significant adhesions to the thoracic wall, a partial (or anterior) capsulectomy can be performed. After capsulectomy, an ADM can be placed to create a dual plane pocket (46). This places the upper two-thirds of the implant below the pectoralis major muscle and the lower third in an epipectoral position. One reason why ADMs reduce the risk of capsular contracture may be the reduced level of bacterial colonization (47).
When there is a pronounced capsular contracture that cannot be successfully treated despite all possible treatments [i.e. capsulectomy with implant reconstruction or rearrangement of the implantation plane from epipectoral to subpectoral (dual plane) with mesh or ADM], patients should be offered capsulectomy with implant removal and autologous tissue replacement (48).
Implant rupture. Intracapsular ruptures are also called silent ruptures. These are often not detected until a routine mammogram or ultrasound (Figure 5). Extracapsular ruptures can trigger local inflammation and cause granuloma formation such as siliconomas (49).

Figure 5.
Intracapsular rupture of the implant – magnetic resonance imaging (left) and intraoperative view (right).
The US Food and Drug Administration recommendation is that women with silicone gel breast implants should undergo mammographic imaging 3 years after surgery and every 2 years thereafter. Magnetic resonance imaging is the most sensitive and specific imaging modality. Overall, the utility of these examinations is questionable because implant ruptures are rare in the first 3 to 5 years after implant placement (50).
When an implant has ruptured it should be removed. Otherwise, inflammation and other tissue reactions may be favored. In this case, a capsulectomy is usually performed to remove the gel material from the breast pocket, especially because there is a possibility of the development of ALCL in women with ruptured textured implants.
Nevertheless, there is no clear evidence that developing silicone granuloma contributes to systemic disease (49).
Breast implant-associated ALCL. This can occur in the scar capsule next to a breast implant filled with silicone or saline. Overall, its incidence in women with breast implants is extremely low. The median time from implantation to breast implant-associated ALCL diagnosis is reported to be 10,3 years (51).
The leading symptom of breast implant-associated ALCL was reported as periprosthetic fluid collection in 86% of patients. In addition, almost all patients had implants with textured surfaces. As of July 2019, 573 individual cases with 33 deaths have been confirmed worldwide (52). Of these cases, 481 were attributed to implants from the manufacturer Allergan. Allergan was also the manufacturer in 12 out of 13 deaths in which the specific manufacturer of the implant was known. No cases of ALCL have been reported in patients with smooth implants. For this reason, the Food and Drug Administration required Allergan to remove all textured implants from the market in July 2019.
Wrinkling and palpability. A common esthetic problem is wrinkling of the implant. This causes skin irregularities that are typically visible at the lateral edge of the breast. This problem is more common in slender patients and with subglandular placement of saline implants. Textured implants are more likely to experience wrinkling and rippling and may be less suitable for patients with thin skin soft-tissue mantles.
In patients with a BMI of less than 18.5 kg/m2, submuscular placement or the use of a silicone implant may be beneficial to minimize the risk of rippling. Saline implants may have the advantage of being less palpable in very slender patients, especially at the lateral and lower edges of the breast. Rippling can also be reduced by placing an ADM in the appropriate area of the breast pocket or by injecting autologous fat grafts (lipofilling).
Breast asymmetry. Asymmetry is related to both the patient’s expectation and the surgeon’s experience. The better the challenges are explained to the patient upfront, the higher the acceptance of the result. When immediate reconstruction is performed, complete adaptation and symmetry of the breasts is often expected by the first postoperative day. Usually, the shape of the adapted breast differs compared to the non-operated breast. Some asymmetries in terms of volume, shape and position of the nipple-areolar complex are also found in almost every non-operated woman. To assess asymmetry, one considers the following aspects: The position of the nipple-areola complex, height of the inframammary fold, and the size and shape of the reconstructed breast compared to the contralateral breast. Meaningful assessment of asymmetry can be performed only from about 3 months after surgery. Photographic documentation to note asymmetries during the initial examination may be useful. This can be helpful for patients to better manage potential changes after reconstruction. Many breast reconstruction patients have a recurring desire for absolute symmetry of both breasts.
Inframammary fold asymmetry can be avoided by correct implant placement. The use of ADM can help to fix the fold at a precise level (e.g., Rhyan’s surgery). Secondary procedures on the contralateral breast may often be required (53). Adjustments in areola position and various mastopexy techniques can resolve asymmetries compared with the opposite side. Postoperative breast asymmetry is more common after unilateral reconstruction than after bilateral procedures, especially after nipple-sparing mastectomy.
- Reconstructive breast surgery following total or partial mastectomy can be performed using autologous tissues or breast implants, and each has its own set of complications.
- Most women do not experience significant complications and are highly satisfied; however, a range of problems can occur any time following reconstructive breast surgery, which are important to consider for women pursuing these options.
- Complications associated with flap-based or implant-based breast reconstruction that may or may not lead to reoperation can be classified as those:
- inherent to surgery and common to all, including seroma, bleeding, and hematoma; skin necrosis; and infection, among others.
- specifically related to the reconstruction, such as flap ischemia/necrosis/loss; fat necrosis; implant capsular contracture; implant failure, exposure, or malposition.
- Failure of silicone gel implants is difficult to detect since the gel typically remains confined within the breast capsule (intracapsular rupture) but in some cases it may extrude into the breast tissue and beyond (extracapsular rupture). Ruptured silicone gel implants should be removed due to the possibility of the gel material causing inflammation and other tissue reactions, particularly when rupture is extracapsular.
- ALCL has been associated with breast implants, located within the scar capsule adjacent to a silicone or saline-filled breast implant. Prophylactic breast implant removal in patients without symptoms or other abnormalities is not recommended.
Reconstructive breast surgery has been a key element of surgical therapy for breast carcinoma for decades. Comprehensive education about potential complications prior to reconstructive surgery is critical. Complications depend on individual patient characteristics, type of mastectomy, multimodality breast cancer therapies (chemotherapy, radiation therapy), and choice of reconstruction method (alloplastic or autologous). Due to the complexity of reconstructive breast surgery, its success is largely dependent on the experience of the surgeon.
A solid training in senology includes reconstructive breast surgery and should continue to enable specialized gynecologic breast surgeons at certified breast centers to recognize, avoid and treat complications. Knowledge of possible pitfalls is essential for the lasting success of breast reconstruction.
Nerve Pain After Latissimus Dorsi Breast Reconstruction
In the past 10 years, continuous advancements have been made in breast reconstruction techniques, in addition to therapeutic mastectomy for breast cancer, while paying careful attention to aesthetics. Among various breast reconstruction techniques, breast reconstruction with the latissimus dorsi (LD) musculocutaneous flap, first introduced in the mid-1970s by Schneider et al. [1] and Olivari [2], has been widely used because of advantages associated with being a relatively simple surgical technique, while overcoming insufficient volume and skin [3].
Needless to say, there are potential postoperative complications, but compared to other surgical techniques, it has a very low risk and incidence rate of complications. Because breast reconstruction with the LD musculocutaneous flap has a relatively smaller surgical range than others, postoperative scars are more favorable that those from other surgical methods, and it also offers a lower risk of complications such as infection and hematoma owing to its relatively shorter operating duration [4].
Complications like seroma, scarring, infection, and breast animation have been reported by other surgeons as a common occurrence. Herein, information is shared on a case of radial nerve neuropathy which occurred for the first time among more than 500 cases of LD flap breast reconstruction performed over the past 8 years at Kyungpook University Medical Center.
Go to : 
CASE
A 51-year-old female patient visited Kyungpook University Medical Center after being diagnosed with ductal carcinoma in situ (DCIS) in the left (Lt.) breast from a biopsy performed at another hospital. The department of surgery scheduled a skin sparing mastectomy (SSM) and the patient was referred to the department of plastic surgery for consultation on breast reconstruction. After consulting with a plastic surgeon, volumetry was performed and the decision was made to use a LD flap breast reconstruction technique suitable for moderate-sized breasts (right [Rt.] 180 cc, Lt. 185 cc) which met the patient’s needs. Afterwards, preoperative photos, chest computed tomography (CT) angiography, and the shoulder’s range of motion (ROM) were assessed according to protocol. The surgery was performed by the surgical department and involved a SSM in the supine position, followed by LD flap elevation and tunneling after changing to the Rt. decubitus position in order to move into the site of skin and soft tissue defects. The patient was returned to the supine position to perform the breast reconstruction while the position of the LD flap was adjusted. There were no specific findings in the surgical sites on the Lt. breast and donor site following the surgery; however, abnormal findings, including numbness, wrist drop and a tingling sensation in the Rt. arm were found starting at postoperative day (POD) 1. Electromyography (EMG) was performed to check for abnormalities, and a difference of ≥20% between the EMG measurements from the Lt. And Rt. sides suggested nerve abnormalities.
In Fig. 1, all cases were determined to be normal since there were no cases showing a difference of ≥20% in latency, latency peak, and amplitude between the Lt. and Rt. sides on the sensory nerve conduction study (NCS) performed on POD 1. However, there were abnormal findings, as shown in Fig. 2, where there was a difference of ≥44% (2.71/1.88*100) in latency values between the Lt. and Rt. sides on the motor NCSs performed on POD1.
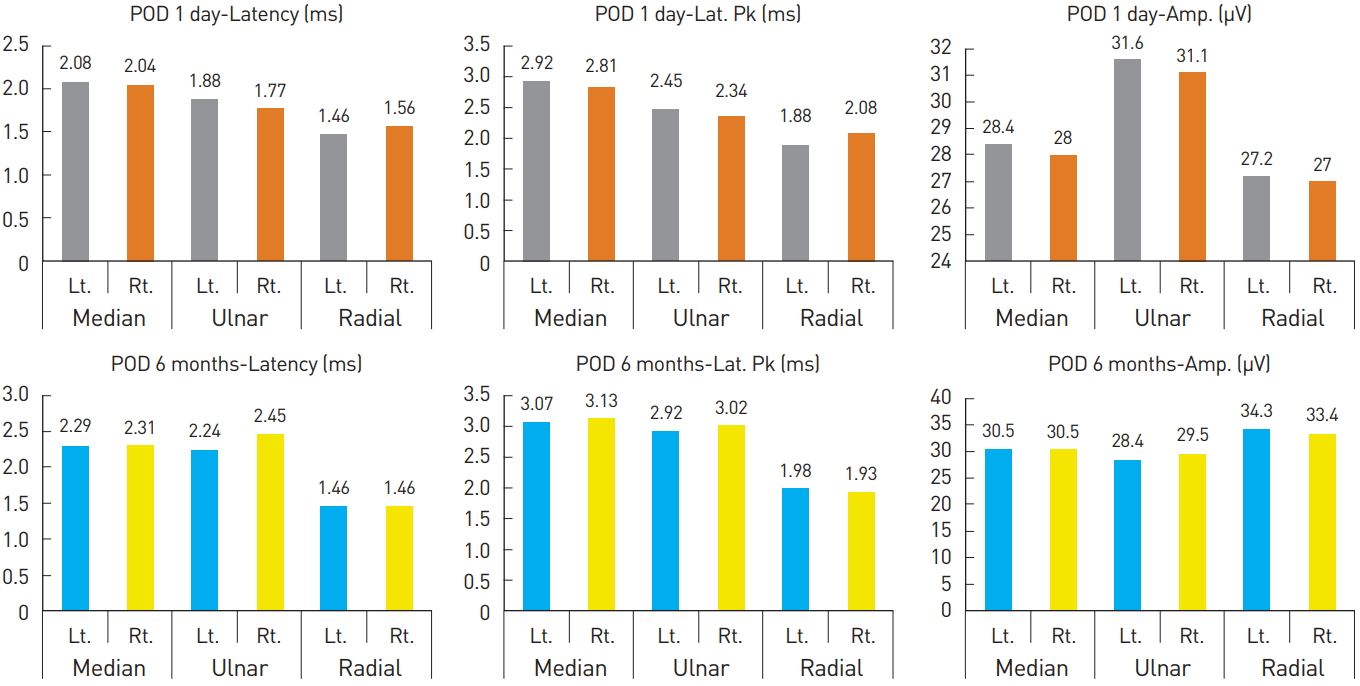 | Fig. 1.Sensory nerve conduction study on postoperative day 1 and at 6 months. POD, postoperative day; Rt., right; Lt., left; Amp, amplitude; Lat. Pk, latency peak. |
 | Fig. 2.Motor nerve conduction study on postoperative day 1 and at 6 months. POD, postoperative day; Rt., right; Lt., left; Amp, amplitude. |
Moreover, abnormal findings in the positive sharp wave (PSW) of the needle EMG appeared in the external carpi radialis longus, external indicis, and brachioradialis of the Rt. arm (Table 1). Considering the muscles showing abnormal findings, a diagnosis was made of radial nerve neuropathy caused by injury to the radial nerve level.
Table 1.
Needle electromyography summary table for postoperative day 1 and at 6 months
POD, postoperative day; MUAP, motor unit action potential; IA, insertion activity; Fib, fibrillation; PSW, polyspike wave; Fasc, fasciculation; H.F., high frequency; Amp, amplitude; Dur., durations; PPP, polyphasic potential; Rt., right; RAD, radialis; N, normal; D, decreased.

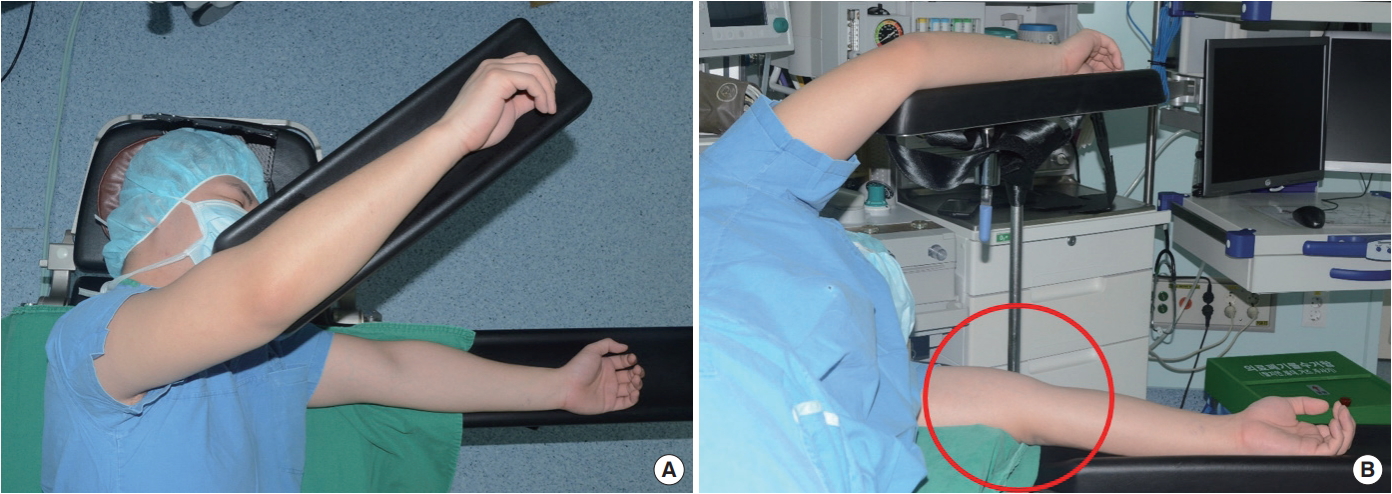
Based on the surface anatomy, it was suspected that compressive radial nerve neuropathy had occurred due to unstable positioning during the LD flap breast reconstruction, which required several position changes during the procedure (Fig. 3).
Afterwards, aggressive pain control with medication (maxnophen 32 mg/37.5 mg qd, gabapentin 300 mg tid), complex exercise, and splint application were implemented with consultation from the department of rehabilitative medicine. As a result, from POD 1 month on, sensory problems including numbness and a tingling sensation were resolved; from POD 3 months on, motor problems including wrist drop also showed improvement; and by POD 6 months, complete recovery was achieved. EMG performed at POD 6 months showed normal findings (Table 1, Fig. 1 and 2), and since then, the patient has shown no further abnormal findings.
Go to : 
DISCUSSION
LD flap breast reconstruction is a procedure that uses autologous tissues and is often used for breast reconstruction following a mastectomy among Asian women with small to moderate-sized breasts [4,5]. The surgical technique has advanced over time with many surgeons choosing to use the procedure, and reports on complications associated with the procedure have been decreasing [6-8]. However, the present case demonstrates that perioperative complications, such as radial nerve neuropathy, can also occur in the opposite arm, not only at the surgical site.
The radial nerve originates from the brachial plexus and supplies the posterior portion of the upper limbs, and is involved in innervation of the 12 muscles responsible for extension of the wrists and hands (Fig. 4A) [9]. Moreover, the superficial radial nerve branch is responsible for sensory functions of the hand dorsum site. The radial nerve is one of the major nerves that is injured most frequently in the upper extremities. Radial nerve neuropathy, sometimes referred to as “honeymoon palsy,” occurs from compression of the arm which injures the radial nerve, causing problems with the supinator and extension of the elbow and wrist. The symptoms may appear differently depending on the level of injury to the radial nerve (Fig. 4B).
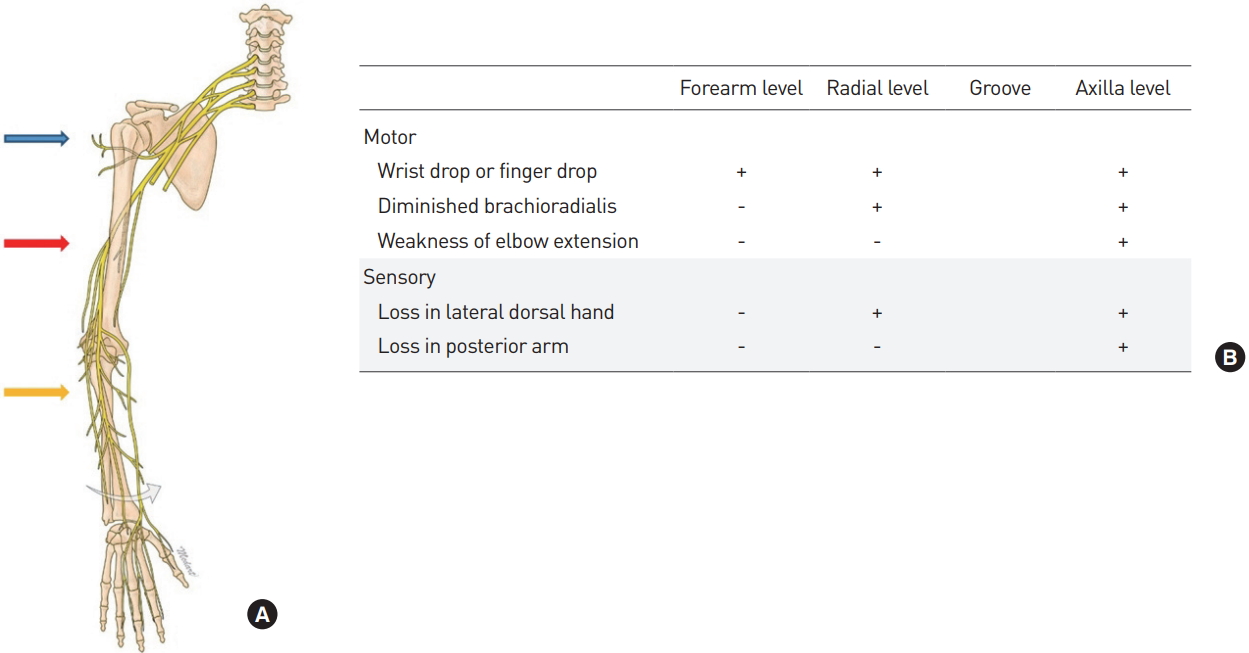 | Fig. 4.(A) Level of radial nerve injury. (B) Symptoms based on injury levels. blue arrow, axilla level; red arrow, radial groove level; yellow arrow, forearm level. |
Nerve injury caused mainly by compression can be classified into demyelinating, axonal, and neurotmesis types (Table 2). Among these, the demyelinating and axonal types, which do not cause nerve continuity problems, have different periods required for recovery depending on the extent of the nerve injury. Moreover, most cases show complete recovery with just conservative treatments such as pain control, complex exercise, and splint application. However, for the neurotmesis type, which causes nerve continuity problems due to trauma (e.g., humerus fractures), recovery with just conservative treatments cannot be expected; thus, aggressive treatments such as surgical interventions are needed, and sequelae can remain after treatment [10].
Table 2.
Nerve injury classification and treatment strategy
| Demyelinating | Axonal | Neurotmesis | |
|---|---|---|---|
| Injury | No loss | Axon | Neural tube |
| Recovery time | 2–4 weeks | Several months | Permanent |
| Treatment | Conservative | Conservative | Surgical intervention |

The present case demonstrated a nerve injury on the opposite arm, unrelated to the surgical site. Thus, peripheral neuropathy such as radial nerve neuropathy can occur at any time if persistent compression is applied to the upper arm by surgical or other devices during the several position changes required in LD flap breast reconstruction. As shown in Fig. 5, gel padding can be used as prevention on the area where compression may occur, and this measure should be able to help prevent such complications from occurring [11].
Looking at the present case from a broader perspective, intraoperative complications can occur not only at surgery-related areas, but also in unexpected areas, such as radial nerve neuropathy in the opposite arm; therefore, close attention should be paid to the smallest of patient-related issues during all surgical procedures.
Chronic Back Pain After Latissimus Dorsi Breast Reconstruction
Background: The latissimus dorsi (LD) flap is used in cases of immediate breast reconstruction after total or partial mastectomy. However, studies on the effect of unilateral LD flap reconstruction on skeletal posture and comparison with results from mastectomy-only have been sparse. Thus in this prospective, observational study, we compared skeletal posture and functional recovery in patients who underwent a mastectomy-only versus those who underwent breast reconstruction with a LD flap after mastectomy.
Methods: From January 2018 to February 2020, a total of 54 patients were enrolled. The control group included 23 patients who underwent mastectomy-only and the experimental group included 31 patients who underwent breast reconstruction using a LD flap immediately after mastectomy. We assessed the Cobb’s angle in spine X-rays, parameters derived from photometry, computed tomography (CT), and 3D scanning preoperatively (T0), 6 months post-surgery (T1), and 1-year post-surgery (T2). We also evaluated functional outcomes, such as pain intensity, disability of the upper extremities, and quality of life.
Results: In the control and experimental groups, the average age was 58.7/46.2 years, body mass index (BMI) was 24.9/22.5, and excised mass weight was 386.8/259.1 g, respectively. In the control group, differences in the Cobb’s angle were significant between T0 and T2 (P=0.003). There were significant differences in the Cobb’s angle and time interaction effects between the two groups (P=0.015). The degree of change in the Cobb’s angle between T0 and T1 was positively correlated with change in the vertical distance from the 3D scanner midline to the nipple (P=0.009).
Conclusions: The experimental group showed improved recovery in skeletal posture compared to the control group. Further, discovering the parameters that can predict the change of skeletal posture through a 3D scanner will have clinical significance. Accordingly, performing breast reconstruction by unilaterally applying the LD muscle is a safe, reliable, and useful method of autologous tissue transfer for breast cancer patients.
Keywords: Mastectomy; mammaplasty; quality of life; surgical flaps